Hf Koh Net Ionic Equation
 | Examples of |
- 1.
- What alkaline earth metal is located in menstruation 3?
- (a) Li
- (b) Na
- (c) Ca
- (d) Mg
- (eastward) Sr
- two.
- Which of the post-obit is classified every bit a metallic?
- (a) Ge
- (b) As
- (c) F
- (d) V
- (e) Ar
- 3.
- Which of the following is a weak acid?
- (a) H2SOfour
- (b) HClO3
- (c) HF
- (d) HCl
- (e) HNOthree
- 4.
- Which one of the following is likely to be the nigh soluble base?
- (a) Ca(OH)2
- (b) Cu(OH)ii
- (c) Ga(OH)3
- (d) Zn(OH)2
- (e) Zr(OH)iii
- 5.
- Which 1 of the following statements is TRUE?
- (a) One mole of any acid will ionize completely in aqueous solution to produce one mole of H+ ions.
- (b) Solutions of weak acids always have lower concentrations of H+ than solutions of stiff acids.
- (c) There are several mutual acids that are insoluble.
- (d) All of the IA and IIA metal hydroxides are soluble.
- (eastward) All weak acids are insoluble.
- vi.
- Consider the post-obit reaction:
NH3(chiliad) + H2O(l) Which one of the following statements is faux?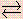 NH4 +(aq) + OH - (aq)
NH4 +(aq) + OH - (aq) - (a) The double arrows bespeak that ammonia, NH3, is simply very slightly soluble in water.
- (b) The reaction is reversible.
- (c) When ammonia is added to water, NH4 + and OH - ions are produced in a 1:ane ratio.
- (d) When solutions of NH4Cl and NaOH are mixed, some ammonia is produced.
- (e) Ammonia is considered to be a weak base of operations.
- 7.
- Which 1 of the following salts is insoluble?
- (a) NH4Cl
- (b) Ca(NO3)2
- (c) BaCOthree
- (d) NaiiS
- (e) Zn(CH3COO)ii
- 8.
- What table salt is formed in the post-obit acrid/base of operations reaction?
HClO3 + Ba(OH)2 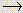
- (a) BaCl2
- (b) ClOBa
- (c) H2O
- (d) BaClOiii
- (e) Ba(ClOthree)2
- nine.
- The precipitate formed when barium chloride is treated with sulfuric acid is _______ .
- (a) BaSiiO4
- (b) BaSO3
- (c) BaSOtwo
- (d) BaSO4
- (east) BaS
- x.
- The spectator ion(s) in the post-obit reaction is/are:
NatwoCOthree(aq) + Ba(NOthree)2(aq) 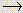 BaCO3(due south) + 2NaNO3(aq)
BaCO3(due south) + 2NaNO3(aq) - (a) Na+ and Baii+
- (b) Ba2+ and CO3 2-
- (c) CO3 2- and NO3 -
- (d) Na+ simply
- (due east) Na+ and NOthree -
- 11.
- Which one of the following statements is Faux?
- (a) For the reaction of a strong acrid with a strong soluble base, the net ionic equation is always
H+ + OH- 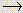 H2O
H2O - (b) "Spectator ions" appear in the total ionic equation for a reaction, only not in the net ionic equation.
- (c) HF, HCl, and HNO3 are all examples of strong acids.
- (d) Titration is a process which tin can be used to make up one's mind the concentration of a solution.
- (due east) In a neutralization reaction, an acid reacts with base of operations to produce a salt and HiiO.
- 12.
- What is the internet ionic equation for the acid-base reaction that occurs when acetic acid and potassium hydroxide solutions are mixed?
- (a) H+(aq) + OH-(aq)
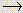 H2O(l)
H2O(l) - (b) H+(aq) + KOH(south)
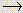 K+(aq) + H2O(50)
K+(aq) + H2O(50) - (c) CH3COOH(aq) + KOH(south)
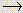 KCHthreeCOO(aq) + H2O(l)
KCHthreeCOO(aq) + H2O(l) - (d) CH3COO-(aq) + H+(aq) + Thou+(aq) + OH-(aq)
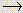 M+(aq) + CHthreeCOO-(aq) + H2O(fifty)
M+(aq) + CHthreeCOO-(aq) + H2O(fifty) - (due east) CH3COOH(aq) + OH-(aq)
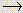 CH3COO-(aq) + H2O(fifty)
CH3COO-(aq) + H2O(fifty) - 13.
- What is the internet ionic equation for the acid-base reaction that occurs when nitric acid is added to copper(Two) hydroxide?
- (a) H+(aq) + OH-(aq)
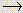 HtwoO(50)
HtwoO(50) - (b) 2H+(aq) + Cu(OH)two(s)
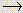 Cutwo+(aq) + 2H2O(fifty)
Cutwo+(aq) + 2H2O(fifty) - (c) 2HNOthree(aq) +Cu(OH)2(s)
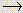 Cu(NO3)two(south) + 2H2O(l)
Cu(NO3)two(south) + 2H2O(l) - (d) 2H+(aq) + 2NO3 -(aq) + Cu2+(aq) + 2OH-(aq)
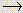 Cu(NO3)2(south) + 2H2O(l)
Cu(NO3)2(south) + 2H2O(l) - (e) 2H+(aq) + 2NO3 -(aq) + Cu2+(aq) + 2OH-(aq)
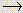 Cutwo+(aq) + 2NOthree -(aq) + 2HtwoO(l)
Cutwo+(aq) + 2NOthree -(aq) + 2HtwoO(l) - 14.
- Which of the post-obit statements is Faux given the following net ionic equation?
H3PO4(aq) + 3OH-(aq) 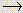 PO4 iii-(aq) + 3HiiO(l)
PO4 iii-(aq) + 3HiiO(l) - (a) If all the h2o evaporated abroad, the common salt remaining could possibly be Na3PO4.
- (b) The acid, H3PO4, is a weak electrolyte.
- (c) The base involved must be a strong soluble base.
- (d) This is classified as a neutralization reaction.
- (e)This could be the net ionic equation for H3PO4 reacting with Al(OH)3.
- 15.
- Which of the following statements is Faux given the following net ionic equation?
2H+(aq) + Cu(OH)2(s) 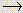 Cu2+(aq) + 2H2O(fifty)
Cu2+(aq) + 2H2O(fifty) - (a) If all the h2o evaporated away, the salt remaining could peradventure exist CuS.
- (b) The acid involved must exist a strong electrolyte.
- (c) The base, Cu(OH)ii, is an insoluble base.
- (d) This could be the net ionic equation for HNO3 reacting with Cu(OH)2.
- (e) This is classified as a neutralization reaction.
- 16.
- Determine the oxidation number of carbon in K2CO3.
- (a) 0
- (b) +2
- (c) +4
- (d) -two
- (e) some other value
- 17.
- Which consignment of oxidation number is Incorrect for the blinking element?
- (a) K2 twoOseven; +6
- (b) H3; +3
- (c) Htwo O2 - ; +i
- (d) O3 ii- ; +four
- (e) (NO3)2; +2
- 18.
- Consider the following reaction:
4NH3 + 5O2 The element beingness oxidized and the oxidizing agent are: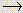 4NO + 6H2O
4NO + 6H2O - (a) N and NHthree
- (b) Due north and O2
- (c) O and NH3
- (d) O and O2
- (e) H and NHthree
- 19.
- Given that the Activity Series is: Na>Mg>Cu>Ag>Au, which 1 of the following answers represents the ions that would not be displaced from aqueous solution (reduced) by metal magnesium?
- (a) Na+
- (b) Cu2+
- (c) Cu2+ and Au+
- (d) Cu2+, Ag+ and Au+
- (e) Na+, Cu2+, Ag+ and Au+
- 20.
- Which proper name/formula combination is WRONG?
- (a) phosphorous acid / H3PO4
- (b) nitrogen oxide / NO
- (c) acetate ion / CHthreeCOO -
- (d) sodium chromate / Na2CrOiv
- (e) calcium hypobromite / Ca(BrO)2
- 21.
- Which name/formula combination is Incorrect?
- (a) chlorous acid / HClO2
- (b) dinitrogen tetroxide / NorthwardiiO4
- (c) ammonium nitrate / NH4NOthree
- (d) copper(II) periodate / CuIO4
- (eastward) potassium permanganate / KMnOfour
Answers:
1. (d) ii. (d) 3. (c) 4. (a) 5. (b) 6. (a) 7. (c) 8. (east) 9. (d) ten. (e) 11. (c) 12. (due east) 13. (b) 14. (e) 15. (a) 16. (c) 17. (b) 18. (b) 19. (a) twenty. (a) 21. (d)

 Click here to render to the top.
Click here to render to the top.
 Choose your side by side chapter:
Choose your side by side chapter: | Atomic Structure | Chemic Periodicity | Chemic Bonding | Molecular Structure/Covalent Bonding Theories| Molecular Orbital Theory |
| Acids/Bases/Salts - Theory & Rxns | Acids/Bases/Salts - Calculations (including balancing redox rxns) | Gases | Solids & Liquids | Solutions |
| Aqueous Equilibrium - Slightly Soluble Salts | Electrochemistry | Metallurgy | Metal Properties & Rxns | Nonmetals & Metalloids |
| Coordination Compounds | Nuclear Chemistry | Organic Chem - Formulas/Names/Properties | Organic Chem - Shapes/Rxns/Biopolymers |

 To report any corrections, please electronic mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please electronic mail Dr. Wendy Keeney-Kennicutt.
Hf Koh Net Ionic Equation,
Source: https://www.chem.tamu.edu/class/fyp/mcquest/ch4.html
Posted by: puckettsectirepas.blogspot.com


0 Response to "Hf Koh Net Ionic Equation"
Post a Comment